One stem cell generates 14 million tumor-killing NK cells in major cancer breakthrough
Researchers in China have created a more efficient strategy for producing natural killer (NK) cells for use in cancer
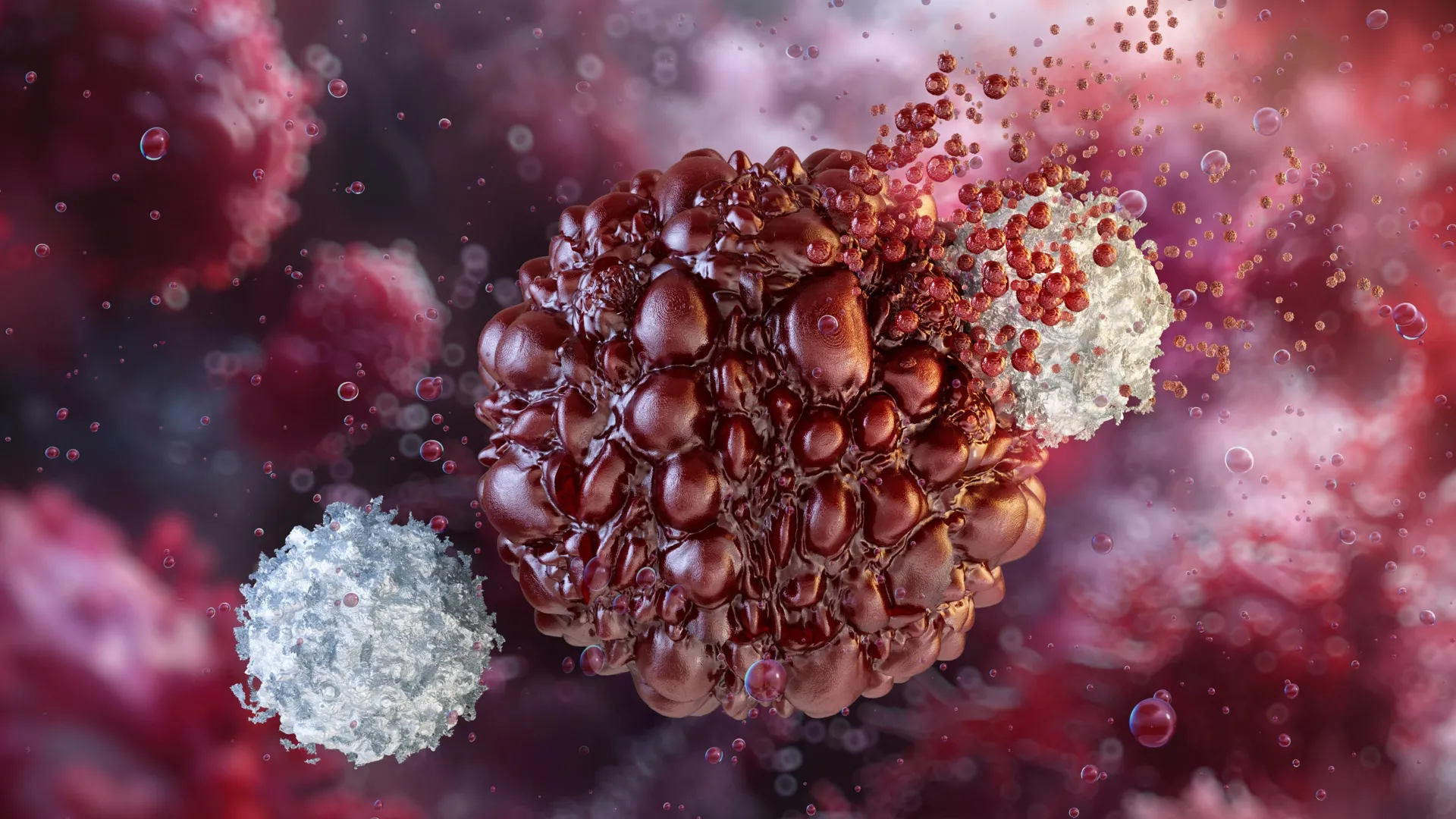
Researchers in China have created a more efficient strategy for producing natural killer (NK) cells for use in cancer immunotherapy.
NK cells play a critical role in the body’s early defense against viruses and cancer, along with other immune functions. Because of their natural ability to detect and destroy abnormal cells, they are an attractive tool for cancer treatment. In chimeric antigen receptor (CAR)-NK therapy, scientists equip NK cells with a lab-designed receptor (a CAR) so they can recognize a specific marker on cancer cells and attack them more precisely.
Traditional CAR-NK approaches usually depend on mature NK cells collected from sources such as peripheral blood or cord blood. This method presents several obstacles, including wide variability between cells, limited efficiency during genetic modification, high production costs, and lengthy preparation times.
Stem Cell-Derived NK Cells From Cord Blood
A team led by Prof. WANG Jinyong at the Institute of Zoology of the Chinese Academy of Sciences developed a different approach. Instead of modifying mature NK cells, the researchers started with CD34+ hematopoietic stem and progenitor cells (HSPCs) taken from cord blood. From these early-stage cells, they generated induced (that is, lab-generated) NK (iNK) cells as well as CAR-engineered iNK (CAR-iNK) cells.
The findings were published in Nature Biomedical Engineering.
Earlier efforts to produce NK cells from cord blood-derived CD34+ HSPCs struggled with low efficiency and immature cell function. To address these limitations, the team moved the genetic engineering step earlier in development, working directly at the CD34+ HSPC stage. This strategy combined CAR transduction, strong expansion of progenitor cells, and guided commitment to the NK lineage.
Three-Step Expansion and Differentiation Process
The researchers used a three-stage system. First, they expanded CD34+ HSPCs (or CD19 CAR-transduced HSPCs) with the help of irradiated AFT024 feeder cells. Within 14 days, the cells multiplied roughly 800- to 1,000-fold.
Next, the expanded cells were cultured with OP9 feeder cells to create artificial hematopoietic organoid aggregates, structures that support efficient NK lineage commitment and development.
In the final stage, cells that had committed to becoming NK cells were allowed to mature and multiply further. This process produced highly pure iNK or CAR-iNK cells that expressed endogenous CD16.
Massive Cell Output From a Single Stem Cell
The team found that a single CD34+ HSPC could generate as many as 14 million iNK cells or 7.6 million CAR-iNK cells. The researchers estimate that one-fifth of a typical cord blood unit could theoretically yield enough cells for thousands or even tens of thousands of treatment doses.
Another major improvement was the sharp reduction in viral vector needed for CAR engineering. Compared with the amount usually required to modify mature NK cells, this method used only about ~1/140,000 (by Day 42 of culture) to ~1/600,000 (by Day 49) as much viral vector.
Strong Tumor Killing in Leukemia Models
In laboratory testing, both iNK and CAR-iNK cells demonstrated powerful tumor-killing ability. In cell line-derived xenograft (CDX) and patient-derived xenograft (PDX) mouse models of human B-cell acute lymphoblastic leukemia (B-ALL), CD19 CAR-iNK cells reduced tumor growth and extended the animals’ survival.
According to the researchers, the new approach not only improves the efficiency of producing iNK and CAR-iNK cells but also significantly lowers the cost of CAR engineering.
The work was supported by the Ministry of Science and Technology of the People’s Republic of China and the National Natural Science Foundation of China, along with other funding sources.