MIT study shows high-fat diets give liver cancer a dangerous head start
A diet high in fat is one of the strongest contributors to liver cancer risk. New research from MIT
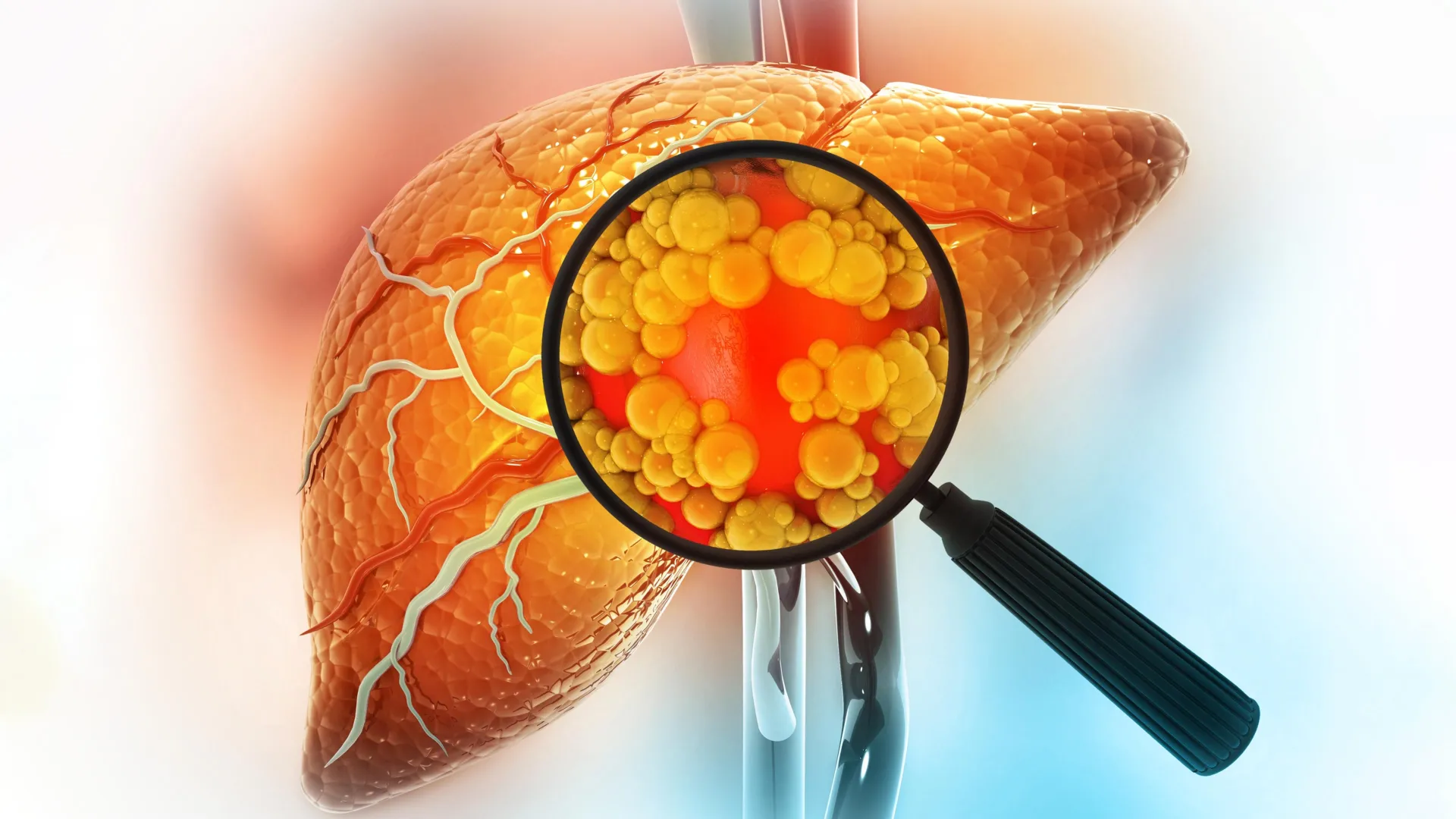
A diet high in fat is one of the strongest contributors to liver cancer risk. New research from MIT sheds light on why this happens, showing that fatty diets can fundamentally alter liver cells in ways that make cancer more likely to develop.
The researchers discovered that when the liver is repeatedly exposed to a high-fat diet, mature liver cells called hepatocytes undergo a major change. Instead of remaining fully specialized, these cells shift into a more primitive, stem-cell-like state. This transformation allows them to better withstand the stress caused by excess fat, but over time it also increases their vulnerability to becoming cancerous.
“If cells are forced to deal with a stressor, such as a high-fat diet, over and over again, they will do things that will help them survive, but at the risk of increased susceptibility to tumorigenesis,” says Alex K. Shalek, director of the Institute for Medical Engineering and Sciences (IMES), the J. W. Kieckhefer Professor in IMES and the Department of Chemistry, and a member of the Koch Institute for Integrative Cancer Research at MIT, the Ragon Institute of MGH, MIT, and Harvard, and the Broad Institute of MIT and Harvard.
The team also identified several transcription factors that appear to regulate this cellular shift. These factors may eventually serve as targets for drugs designed to reduce the risk of tumor formation in people who are especially vulnerable.
Shalek; Ömer Yilmaz, an MIT associate professor of biology and a member of the Koch Institute; and Wolfram Goessling, co-director of the Harvard-MIT Program in Health Sciences and Technology, are the senior authors of the study, which was published on December 22 in Cell. MIT graduate student Constantine Tzouanas, former MIT postdoc Jessica Shay, and Massachusetts General Brigham postdoc Marc Sherman are the co-first authors of the paper.
How Fatty Diets Trigger Liver Cell Reversion
High-fat diets can cause inflammation and fat accumulation in the liver, leading to a condition known as steatotic liver disease. This disease may also arise from long-term metabolic stressors such as heavy alcohol consumption and can progress to cirrhosis, liver failure, and ultimately cancer.
In this study, the researchers set out to understand how liver cells respond at a molecular level when exposed to a high-fat diet, focusing on which genes become more or less active as the stress continues.
To investigate this process, the team fed mice a high-fat diet and used single-cell RNA-sequencing to analyze liver cells at key stages of disease development. This approach allowed them to follow changes in gene activity as the animals progressed from liver inflammation to tissue scarring and eventually cancer.
Early on, hepatocytes began activating genes that help cells survive harsh conditions. These included genes that reduce the likelihood of programmed cell death and promote continued cell growth. At the same time, genes essential for normal liver function, including those involved in metabolism and protein secretion, were gradually shut down.
“This really looks like a trade-off, prioritizing what’s good for the individual cell to stay alive in a stressful environment, at the expense of what the collective tissue should be doing,” Tzouanas says.
Some of these genetic shifts occurred quickly, while others unfolded more slowly. The decline in metabolic enzyme production, for example, developed over a longer period. By the end of the study, nearly all mice fed a high-fat diet had developed liver cancer.
Why Immature Liver Cells Fuel Cancer Development
The researchers found that when liver cells exist in a less mature state, they are more likely to become cancerous if a damaging mutation occurs later.
“These cells have already turned on the same genes that they’re going to need to become cancerous. They’ve already shifted away from the mature identity that would otherwise drag down their ability to proliferate,” Tzouanas says. “Once a cell picks up the wrong mutation, then it’s really off to the races and they’ve already gotten a head start on some of those hallmarks of cancer.”
The study also highlighted several genes that appear to coordinate the shift back to an immature cell state. During the course of the research, a drug targeting one of these genes (thyroid hormone receptor) received approval to treat a severe form of steatotic liver disease known as MASH fibrosis. In addition, a drug that activates another enzyme identified in the study (HMGCS2) is currently being tested in clinical trials for steatotic liver disease.
Another promising target uncovered by the research is a transcription factor called SOX4. This factor is usually active during fetal development and in a limited number of adult tissues (but not the liver), making its activation in liver cells particularly notable.
Evidence From Human Liver Disease
After identifying these cellular changes in mice, the researchers examined whether similar patterns occur in people with liver disease. They analyzed liver tissue samples from patients at different stages of disease, including individuals who had not yet developed cancer.
The results closely mirrored what was seen in mice. Over time, genes required for normal liver function declined, while genes linked to immature cell states increased. The researchers also found that these gene expression patterns could be used to predict patient survival outcomes.
“Patients who had higher expression of these pro-cell-survival genes that are turned on with high-fat diet survived for less time after tumors developed,” Tzouanas says. “And if a patient has lower expression of genes that support the functions that the liver normally performs, they also survive for less time.”
While mice developed cancer within about a year, the researchers estimate that the same process in humans likely unfolds over a much longer period, potentially around 20 years. The exact timeline can vary depending on diet and other risk factors, including alcohol use and viral infections, which can also push liver cells toward an immature state.
Can Diet-Driven Damage Be Reversed?
The research team now plans to explore whether the cellular changes caused by high-fat diets can be undone. Future studies will test whether returning to a healthier diet or using weight-loss medications such as GLP-1 agonists can restore normal liver cell behavior.
They also aim to further investigate whether the transcription factors identified in the study could serve as effective drug targets to prevent damaged liver tissue from progressing to cancer.
“We now have all these new molecular targets and a better understanding of what is underlying the biology, which could give us new angles to improve outcomes for patients,” Shalek says.
The research was funded, in part, by a Fannie and John Hertz Foundation Fellowship, a National Science Foundation Graduate Research Fellowship, the National Institutes of Health, and the MIT Stem Cell Initiative through Foundation MIT.